
Protons and neutrons vibrate, but are basically motionless. Neutrons act as a type of “insulation” between the protons, preventing them from repelling each other. Protons and neutrons (and therefore all of the mass) are located in the center of the atom in a region called the nucleus. Ions can be either negatively charged if they have more electrons than protons, or they can be positively charged if they have more protons than electrons. If the number of protons and electrons is not equal, then it is referred to as an ion. The entire atomic mass is made up of only protons and neutrons and therefore the number of neutrons can be found by taking the mass and subtracting the # of protons.Īn electrically neutral atom will always have the same number of positively charged protons and negatively charged electrons.
The average atomic masses given on the periodic table are a weighted average of the different naturally occurring isotopes of an element.Ī weighted average can be calculated by taking the sum of each isotopic percentage multiplied by the isotopic mass: Atomic mass = (isotope 1 %) x (isotope 1 mass) + (isotope 2 %) x (isotope 2 mass) +. Atomic Mass= # of protons + # of neutrons Atoms of an element which have a different atomic mass are called Isotopes. The chemical properties may vary slightly but will be very similar. The number of neutrons can vary and will affect the mass, but not the identity, of an atom. Electrons have a charge of -1 and a relative mass of 0 AMU. Neutrons have a charge of 0 and a relative mass of 1 AMU.
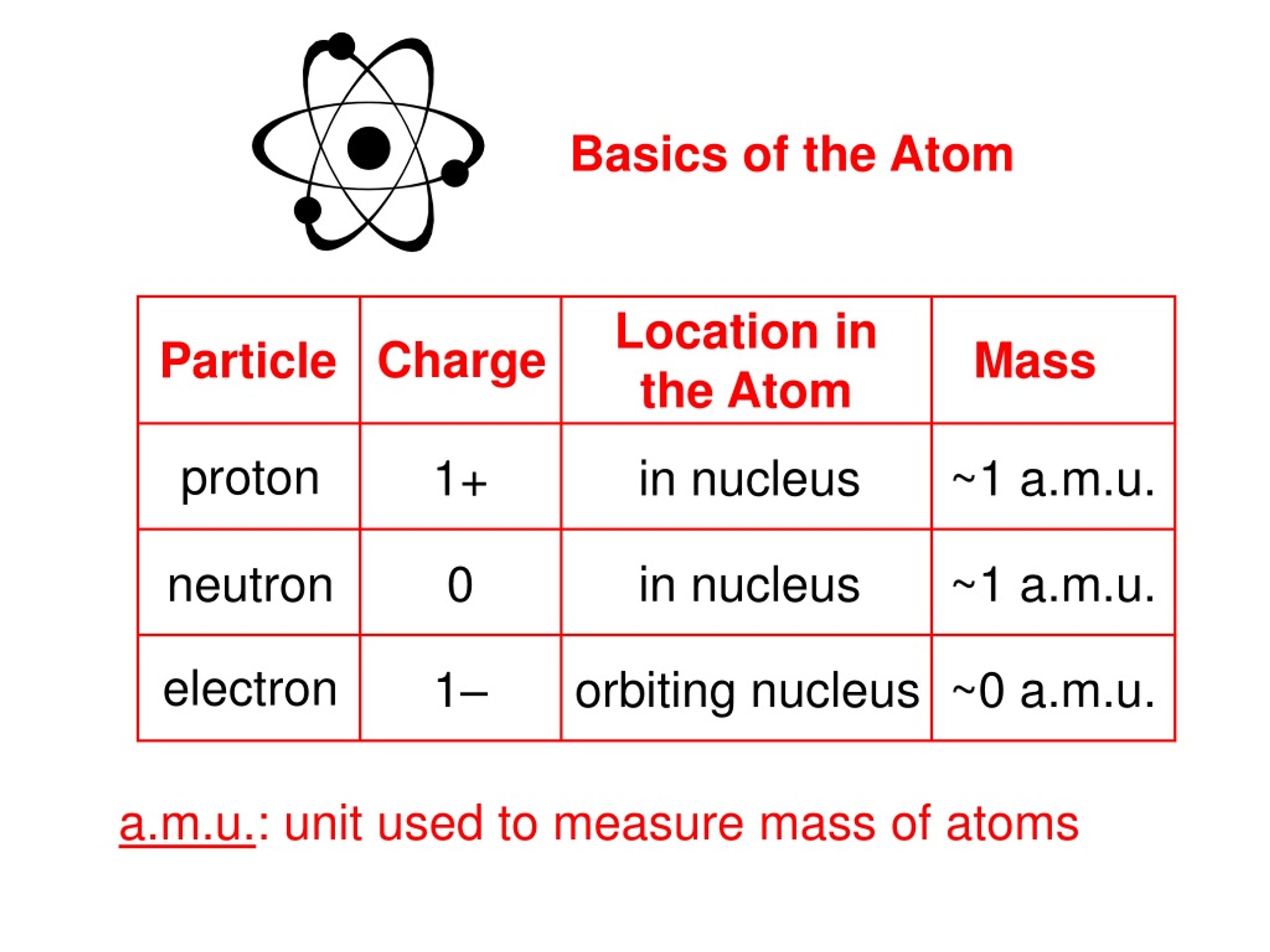
Atoms are the building blocks of matter, sort of how bricks are the building. Protons have a charge of +1 and a relative mass of 1 AMU. Matter is anything that takes up space and has mass. Chadwicks research greatly increased understanding of the structure of the atomic nucleus. There are currently discovered atoms that contain up to 118 protons. James Chadwick 1932 James Chadwick proved the existence of neutrons in 1932. The number of protons in an atom determines the identity and to a large extent the chemical properties of an atom The number of protons is known as the Atomic NumberĪll atoms which have the same number of protons will have very similar chemical properties and are considered the same element. It is the way in which these subatomic particles are put together which determine the properties and type of atom that is formed There are a number of different subatomic particles of which there are three that we concern ourselves with: Protons Neutrons Electrons Presentation on theme: "Atomic Structure Notes"- Presentation transcript:Īn atom is not the smallest particle of matter Atoms are the smallest type of unique matter All atoms are made up of subatomic particles which are identical in all atoms PPT for the whole of the first unit based on the draft SoW from AQA.


 0 kommentar(er)
0 kommentar(er)
